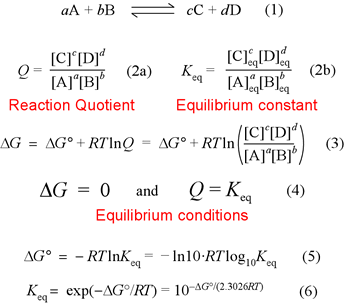
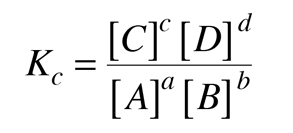![Given the equilibrium constant, Kc of the reaction : Cu(s) + 2Ag^ + (aq) → Cu^2 + (aq) + 2Ag(s) is 10 × 10^15 , calculate the E^ocell of this reaction at 298 K [2.303 RTF at 298 K = 0.059 V ] Given the equilibrium constant, Kc of the reaction : Cu(s) + 2Ag^ + (aq) → Cu^2 + (aq) + 2Ag(s) is 10 × 10^15 , calculate the E^ocell of this reaction at 298 K [2.303 RTF at 298 K = 0.059 V ]](https://dwes9vv9u0550.cloudfront.net/images/1973415/593bf337-c5c4-43f4-b13e-0050160d57a2.jpg)
Given the equilibrium constant, Kc of the reaction : Cu(s) + 2Ag^ + (aq) → Cu^2 + (aq) + 2Ag(s) is 10 × 10^15 , calculate the E^ocell of this reaction at 298 K [2.303 RTF at 298 K = 0.059 V ]

Calculate the equilibrium constant for the the reaction given below at 400K, if Delta H ^(@) = 77.2 kJ mol ^(-1) and Delta S ^(@) = 122JK ^(-1) mol ^(-1) , PCl (s (s)) to PCl (3 (g)) + Cl (2 (g))

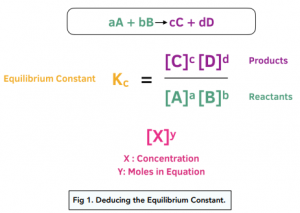
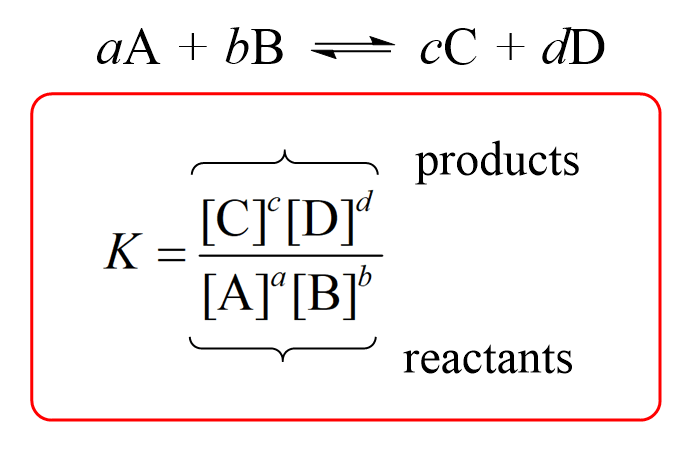
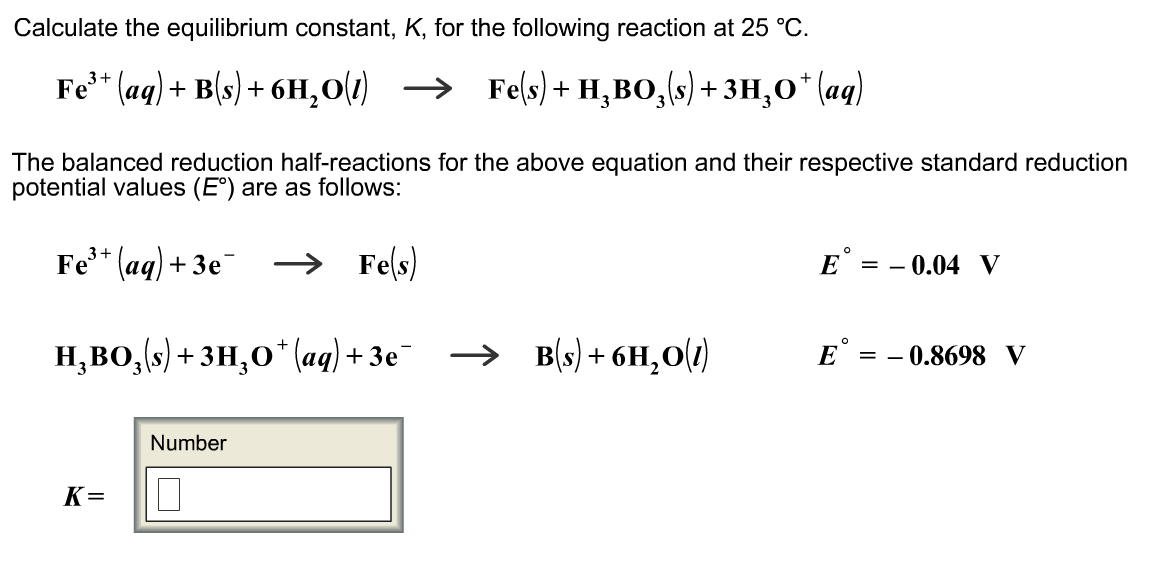
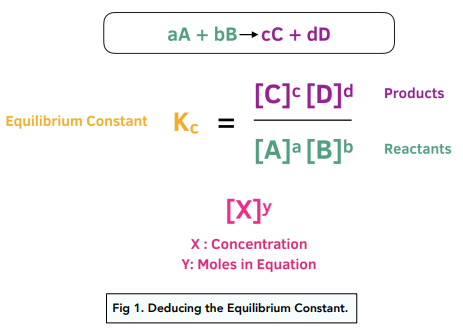
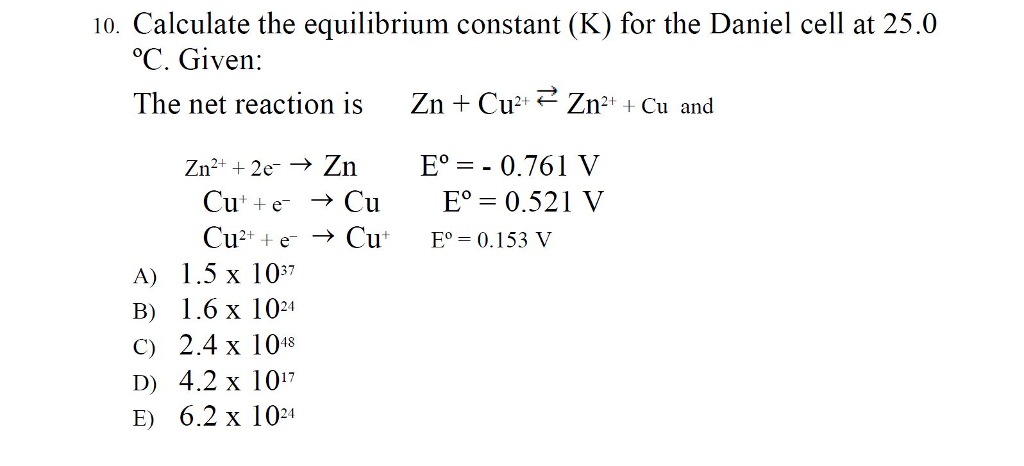
7.2 The Position of Equilibrium.. Assessment Statements Deduce the equilibrium constant expression (K c ) from the equation for a homogeneous reaction. - ppt download
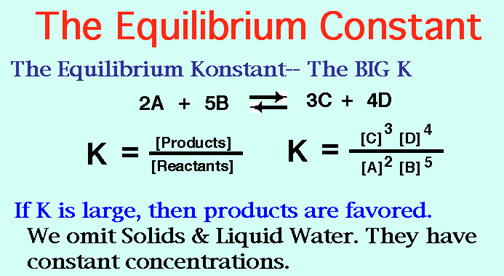
How does the value of an equilibrium constant relate to the relative quantities of reactants and products at equilibrium? | Socratic

Easy tricks to calculate equilibrium constant based problems/Chemical eq... | Simple tricks, Equilibrium, Problem

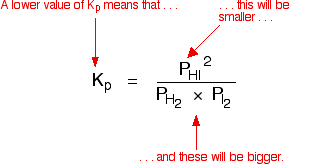
Question Video: Calculating the Equilibrium Constant for Partial Pressures Given the Partial Pressure of Each Species | Nagwa